top of page

123-456-7890
THE AMAZING ELEMENT ENCYCLOPEDIA
-
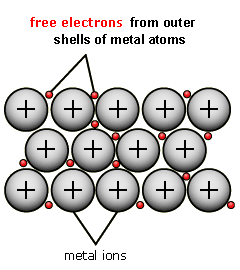
A metallic bond is when metal ions are held together by strong intermolecular forces with seas of freely moving electrons.
-
Metallic bonds have strong intermolecular forces, they have high melting points and boiling points making them hard to break apart.
-
Because of the freely moving electrons, those electrons are able to carry electrical charges around making metals good conductors of electricity.
Metallic Bond
bottom of page