top of page

123-456-7890
THE AMAZING ELEMENT ENCYCLOPEDIA
DROP
THE BASE
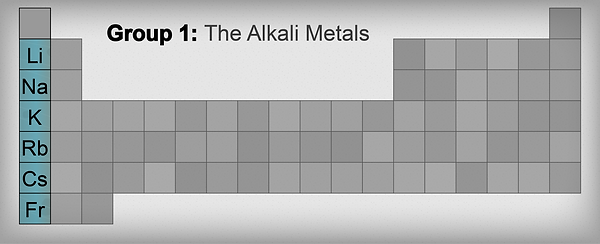
-
Because of their highly reactive nature with air and water, alkali metals are usually stored in oil.
-
Alkali metals have low densities make them able to float on water and they can be easily cut with a knife.
-
They react with water in which it produces hydrogen gas, it gets more reactive and explosive as it goes down the group.
-
They have low boiling points, they also have low melting points that decreases as it goes down the group.
-
Alkali Metals form positive ions by losing an electron to another atom which will be a negative ion through ionic bonding.
bottom of page